LFP48280 48V 280Ah 14.3kWh Lithium Battery Powerwall
48V 280Ah LiFePO4 Battery | High-Capacity Energy Storage with 6000+ Cycles & UL Certification
Key Information
- Technical Highlights:
- Maximum Current: 200A continuous operation.
- Voltage Ranges:
- Recommended Charging: 56.0V ±0.5V
- Discharge Cut-off: 48V ±0.2V
- Low Internal Resistance: ≤40mΩ (AC), enhancing efficiency and reducing energy loss.
- Applications:
-
- Solar energy storage systems
- Backup power for telecom, data centers, and critical infrastructure
- Off-grid and hybrid renewable energy setups
- Commercial/Industrial UPS solutions
SEND INQUIRY

Back to Product List
Elevate your energy resilience with the A11-015KCAA—where power, longevity, and safety converge.
The A11-015KCAA is a high-performance, long-cycle-life lithium iron phosphate (LiFePO4) battery designed for reliable and scalable energy storage solutions. With a robust 14.336kWh nominal energy capacity and advanced safety certifications, this battery is engineered to meet the demands of residential, commercial, and industrial applications.
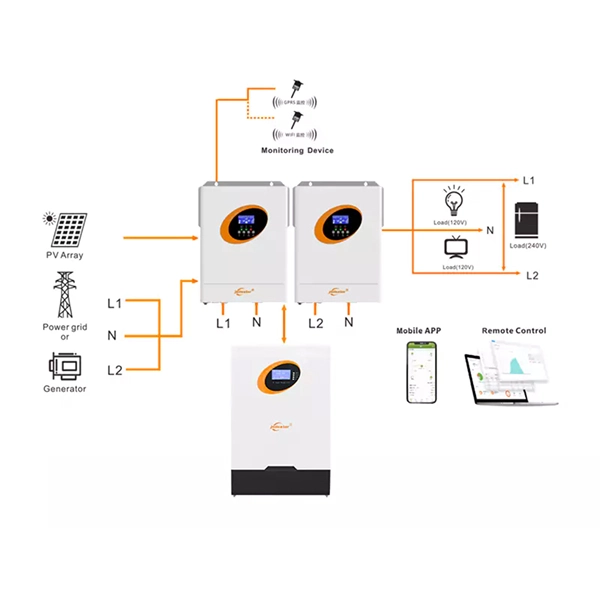
Application Diagram
Product Details
| Model | A11-015KCAA |
| Nominal Voltage | 51.2V(1P16S) |
| Nominal Capacity | 280Ah |
| Nominal Energy | 14.336kwh |
| Internal Resistance (AC) | ≤40mΩ |
| Cycle Life | ≥6000 cycles @ 80% DOD, 25℃, 0.5C;≥5000 cycles @ 80% DOD, 40℃, 0.5C |
| Designed Life | ≥5 years |
| Recommended Charging Voltage | 56.0V±0.5V |
| Maximum Operating Current | 200A |
| Discharge Cut-off Voltage | 48V±0.2V |
| Charging Temperature Range | 0℃–50℃ |
| Discharge Temperature Range | -20℃–55℃ |
| Storage Temperature Range | -20℃~45℃ @ 60%±25% relative humidity |
| Dimensions (LWHmm) | 908470262 |
| Weight | 125kg |
| Communication Mode | RS232-PC, RS485(B)-PC;RS485(A)-Inverter, Canbus-Inverter |
| Number of Parallel Connections | Supports up to 16 units in parallel (6 batteries in parallel recommended) |
| Certification | UL1973, UL9540A, CE-EMC, UN38.3 |
Solar Energy Cases for Different Residential
Common Questions
-
What certifications are required for OEM residential solar products in international markets? 1
-
Our products are designed to assist in drug formulation and quality control, offering solutions for oral, transdermal, and subcutaneous dosage forms. We focus on providing accurate data for drug release, absorption, and overall product stability. 1
-
What certifications are required for OEM residential solar products in international markets? 2
-
Our products are designed to assist in drug formulation and quality control, offering solutions for oral, transdermal, and subcutaneous dosage forms. We focus on providing accurate data for drug release, absorption, and overall product stability. 2
-
What certifications are required for OEM residential solar products in international markets? 3
-
Our products are designed to assist in drug formulation and quality control, offering solutions for oral, transdermal, and subcutaneous dosage forms. We focus on providing accurate data for drug release, absorption, and overall product stability. 3
-
What certifications are required for OEM residential solar products in international markets? 4
-
Our products are designed to assist in drug formulation and quality control, offering solutions for oral, transdermal, and subcutaneous dosage forms. We focus on providing accurate data for drug release, absorption, and overall product stability. 4
-
What certifications are required for OEM residential solar products in international markets? 5
-
Our products are designed to assist in drug formulation and quality control, offering solutions for oral, transdermal, and subcutaneous dosage forms. We focus on providing accurate data for drug release, absorption, and overall product stability. 5
-
What certifications are required for OEM residential solar products in international markets? 6
-
Our products are designed to assist in drug formulation and quality control, offering solutions for oral, transdermal, and subcutaneous dosage forms. We focus on providing accurate data for drug release, absorption, and overall product stability. 6
-
What certifications are required for OEM residential solar products in international markets? 7
-
Our products are designed to assist in drug formulation and quality control, offering solutions for oral, transdermal, and subcutaneous dosage forms. We focus on providing accurate data for drug release, absorption, and overall product stability. 7
Get Free Quote
Our sale team will give full support and price you needed. We are more than happy to answer your questions within 24 hours.
SEND INQUIRY